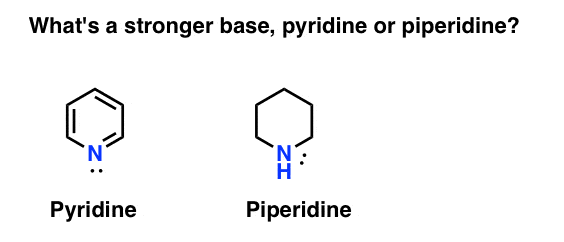
Which among the following is strongest base in gas phase? (a) Triethylamine (b) Diethylamine (c) Ethylamine (d) Ammonia
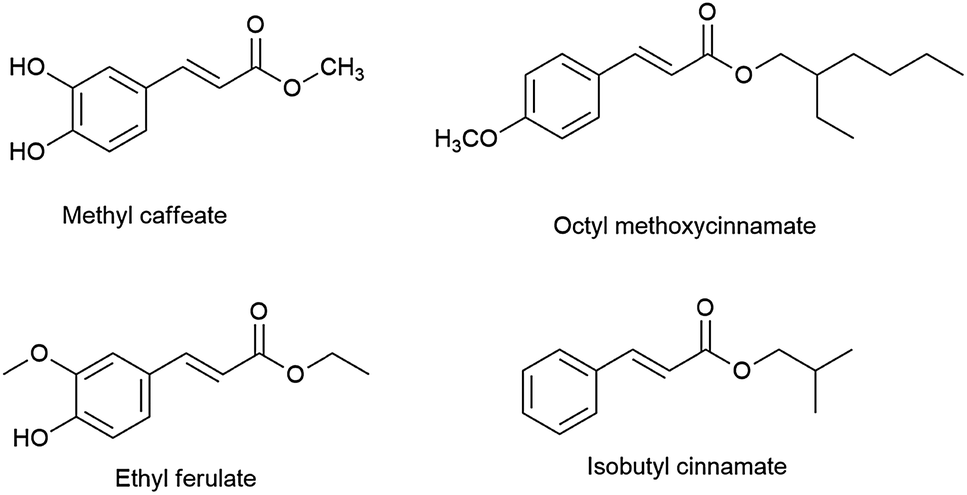
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C5NJ03125G
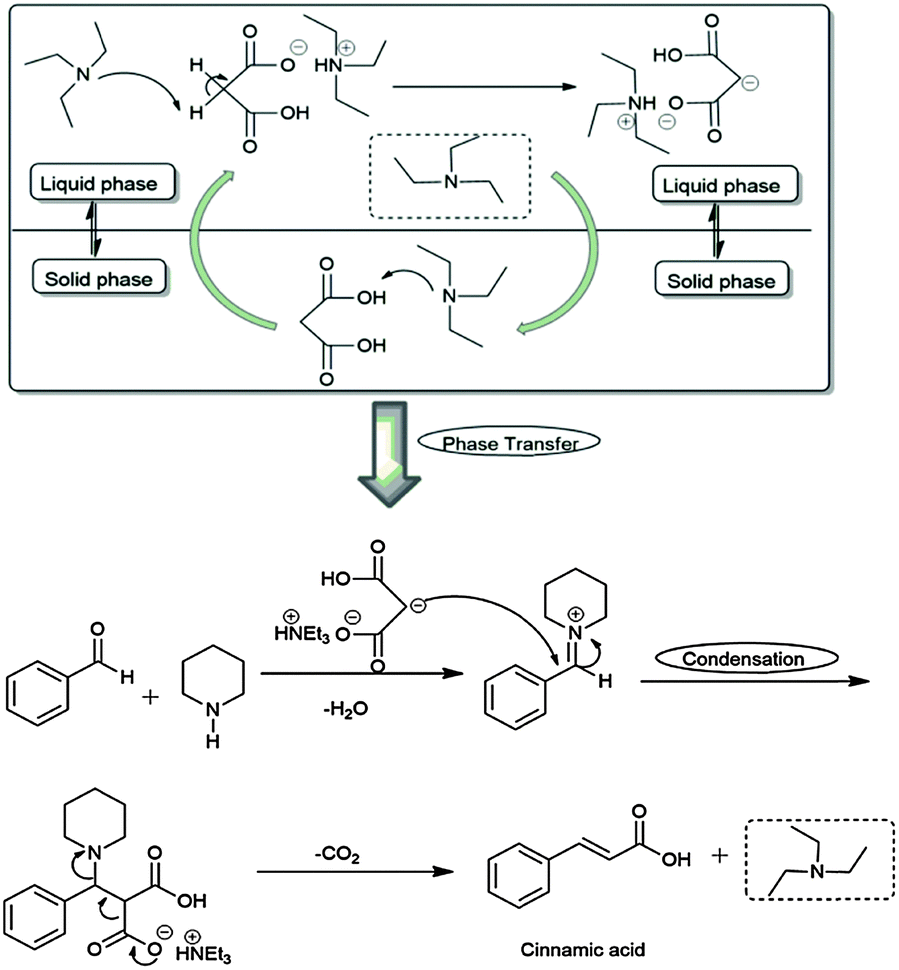
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C5NJ03125G
Triethylamine Organic Base Molecule. Skeletal Formula. Stock Vector - Illustration of drawing, triethyl: 187167254